Some irrigation water and fertiliser product combinations can cause detrimental reactions such as corrosion and sedimentation (resulting in blockages). It is therefore recommended that a jar test is first done by adding the fertiliser at the correct concentration to the irrigation water.
Monitor the mixture for sediments or milkiness over a period of one or two hours. Milkiness will be an indication that blockage problems are possible. Secondary filters at each block can protect the emitters against potential damage.
We thank the ARC Agricultural Engineering in South Africa for making their manual on fertigation principles and systems available to the readers of ProAgri Zambia.

Milkiness in a fertiliser and water solution indicates that blockages may occur. Photo: edis.ifas.ufl.edu.
With the choice of fertiliser products, the following must be kept in mind:
- When a dry water-soluble fertiliser is used, first fill the tanks halfway to three quarters with water and then slowly add the fertiliser while stirring the water continuously to prevent the forming of large, insoluble lumps. Always add the liquid fertiliser to the water in the tank before the dry water-soluble fertiliser is added, to ensure the solution of all the fertiliser products.
- Fertiliser products with an acidic base are inclined to cause corrosion of metal and asbestos cement components of an irrigation system. Ensure that the injection and irrigation equipment are resistant to these products. uPVC, polyethylene, polystyrene, Teflon and Grade 316 stainless steel are the most corrosion-resistant commercial materials. Equipment must also be flushed well after use. Furthermore, the lowest possible concentration of fertiliser must be used.
- Not all fertiliser products are compatible in concentrate form, for example products that contain sulphates are incompatible with products that contain calcium. The result will be the forming of insoluble gypsum. Phosphates are also incompatible with products containing calcium and magnesium. These products must be injected separately from different tanks into an irrigation pipeline (See Figure 1).
This separation will prevent the deposition of calcium phosphates and calcium sulphates in the mixing tank and irrigation system. Injection from the two tanks must take place at least 0,5 m apart. An extra tank is used when acid for the pH-correction of the irrigation water has to be added. When concentrate products are dissolved in tanks, the acids must be mixed in first, then the neutrals and then the alkaline products.
- The different soil and irrigation water combinations can have an acid or alkaline reaction within the root zone. By using specific fertiliser products, this reaction can be counteracted.
- The solubility of dry water-soluble fertiliser products increases as the water temperature rises. Although nitrate-containing products are highly soluble, the water temperature can drop with up to 20°C. This reduction has the result that three times more water is required for dissolving the product than at normal temperatures. This means that the injector’s capacity must also increase threefold.
- Do not mix chlorine and fertiliser, as it will lead to an explosion. Always clean the mixing tank before chlorine is used. The presence of ammonia and urea in the irrigation water will decrease the effectiveness of the chlorine. Chlorine and acid or an acid-based fertiliser product must also not be mixed, because a toxic chlorine gas will form. Always add acid to water, not water to acid, because a massive exothermic reaction can take place which will make the liquid splash out. The same applies to chlorine, always add the chlorine to the water.

Stir the water continuously while adding the fertiliser. Photo: Youtube.com
If irrigation water of a neutral to alkaline nature is used for irrigation, acid correction must first be done before calcium and phosphates are applied, for example high pH values (> 7,5) in the irrigation water can result in the deposition of calcium and magnesium carbonates and phosphates that could lead to emitter blockages. The pH correction of the irrigation water will improve the absorbency of the nutrients, and will prevent the deposition of impermeable compounds thereof. The ideal pH of fertigation is between 5,6 and 6,2. Some of the most important products and the plant nutrient elements they contain, are shown in Table 1.
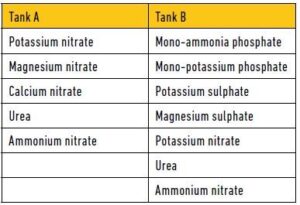
Figure 1: Permissible combinations of fertiliser solutions (Schneck, 1998).
Water soluble fertiliser
To apply fertiliser in granular or powder form, it must be water soluble. The quantity of fertiliser that dissolves in a certain volume of water is called the solubility of the mixture. It is recommended that a professional in the field of fertigation be approached on the solubility and miscibility of a specific fertiliser product. A jar test must also be done.

Precipitation of calcium and magnesium carbonates and phosphates could lead to emitter blockages. Photo: orbitbharat.com.
Liquid fertiliser
Fertiliser products in liquid form can be divided into three groups (Buys, 1997):
(i) Slurries
This group is seldom if ever produced. It can be compared to a porridge or thick lime solution used for painting walls. In such a solution, a reasonable quantity of solid particles occur, that can settle within hours.
(ii) Suspensions
This group can be compared to the lime water after the large lumps have settled. Settling can however still occur, but it can take weeks or months.
There are currently two types of suspensions:

Jar test. Photo: appslabs.com.au.
Nitro-phosphate suspensions
These suspensions are manufactured by the treatment of rock phosphate with nitric acid. The initial product can then be further treated to deliver various end products. The relationship of N:P in the products is 3:2 or 4:1. Nitrophosphates have a high density and are considered heavy suspensions.
Ammonia phosphate suspensions
These suspensions are manufactured by the reaction of phosphorous with ammonia under controlled conditions. The duration of the reaction and the eventual pH will determine the end product. It can contain a MAP, DAP or both and the N:P-relation is between 2:3 and 3:4.
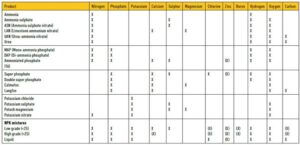
Table 1: Most important fertiliser products (Buys, 1997).
(iii) Solutions
In this group, all the components are dissolved entirely. With the exception of N-solution, this group is characterised by low concentrations, seldom higher than 15%. It is mainly as a result of limitations regarding solubility and the necessity to store such products at room temperature without it crystallising. The disadvantage is that transport costs are noticeably higher than for suspensions.
Clear solutions were mainly developed for irrigation farmers to enable them to apply fertilisers through the irrigation system. The accuracy of application and the frequency with which it is done on specialised crops, compensates for the increased costs because of the lower concentration of clear solutions.
Visit www.arc.agric.za for more information. Next month we shall discuss what fertiliser products to choose.









