This overview aims to explain the utility and use of KynoPlus to arable farmers. As is well known, there are different nitrogen sources to be used in agricultural practices; either during planting, as a pre-plant application, side fertilisation, or top fertilisation. You can even choose between dry fertiliser (granules) or liquid fertiliser. The choices and combinations are ample.
For a long time there has been debate about the best single nitrogen source, whether calcium ammonium nitrate (CAN), urea, or ammonium sulphate (AS). The building blocks of the granular products therefore include ammonium nitrate (CAN), ammonium (AS), or the amide (urea) form. In liquid it involves exactly the same building blocks. Each has unique and fixed characteristics.
Reasons for nitrogen fertilisation are mostly repetitive and typical in nature. Regardless of the crop, nitrogen is applied to the soil to support crop growth to such an extent that a yield (usually according to target) can be obtained. In other words, regardless of whether the crop is pasture, wheat, corn, sunflower, peanut, soybean, alfalfa, or vegetables, there is always a pattern of use, namely soil preparation, pre-fertiliser application if appropriate, planting with fertiliser, possible supplemental fertilisation, and then finally, harvest (excluding weed, pest and pest control).
Pasture crops’ sequence and harvest differs slightly from the other crops in that they can be perennial and are managed and utilised differently. Permanent crops also differ from the first-mentioned group of crops, in that they are established, and then maintained, with a harvest that can be harvested every year (after the crop has come to fruition).
An old saying goes that “all roads lead to Rome”. The reality of all nitrogen sources is that their end product is all the same. See the illustration of the nitrogen cycle in Figure 1, below.
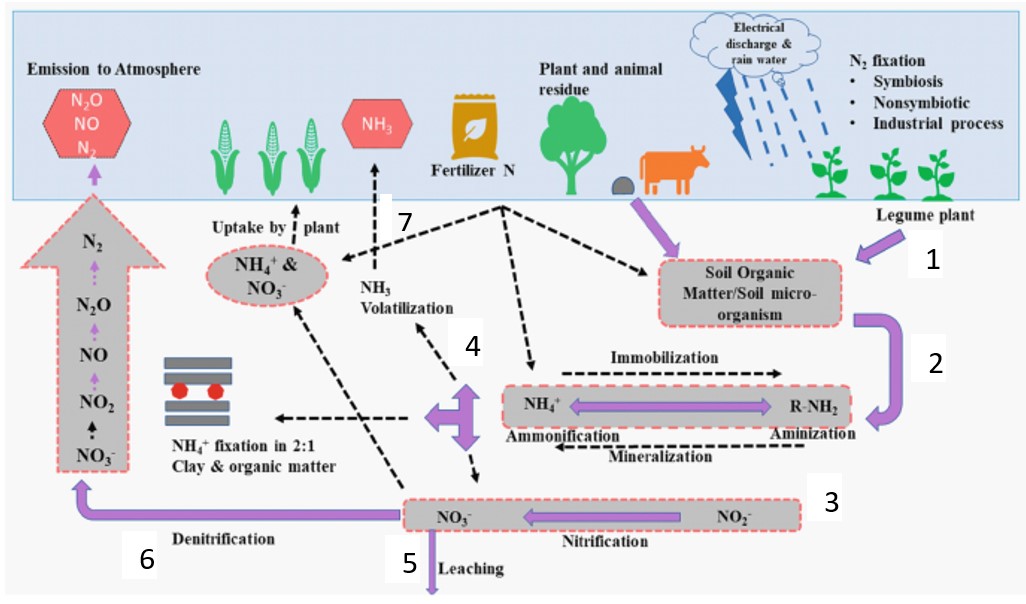
Figure 1: The nitrogen cycle. (Source: Springer Link)
In step 1 of the nitrogen cycle (Figure 1), nitrogen, trapped in plant and animal remains, as well as from the atmosphere during rain associated with lightning, is added to the soil. In step 2, organic nitrogen in the organic residues in the soil is mineralised to ammonium (NH4+) by microorganisms. During step 3 (nitrification), ammonium is converted to nitrate (NO3-) by nitrifying bacteria.
In step 4, nitrate and ammonium are taken up by plant roots to produce the protein found in the crops. In step 5, some of the nitrate may be lost due to leaching along with percolation water. It connects to groundwater that is found in deeper soil layers, or elsewhere. In step 6, some nitrate can be converted by denitrifying bacteria to N2 and nitrogen oxides (N2O and NO) which escapes to the atmosphere. The last step thus completes the cycle.
In step 7, part of the ammonium can be converted to ammonia (NH3) to volatilise into the atmosphere (Havlin et al., 1999). All the above steps therefore describe the concept of additions, changes, and losses; the components of the nitrogen cycle (Figure 2).
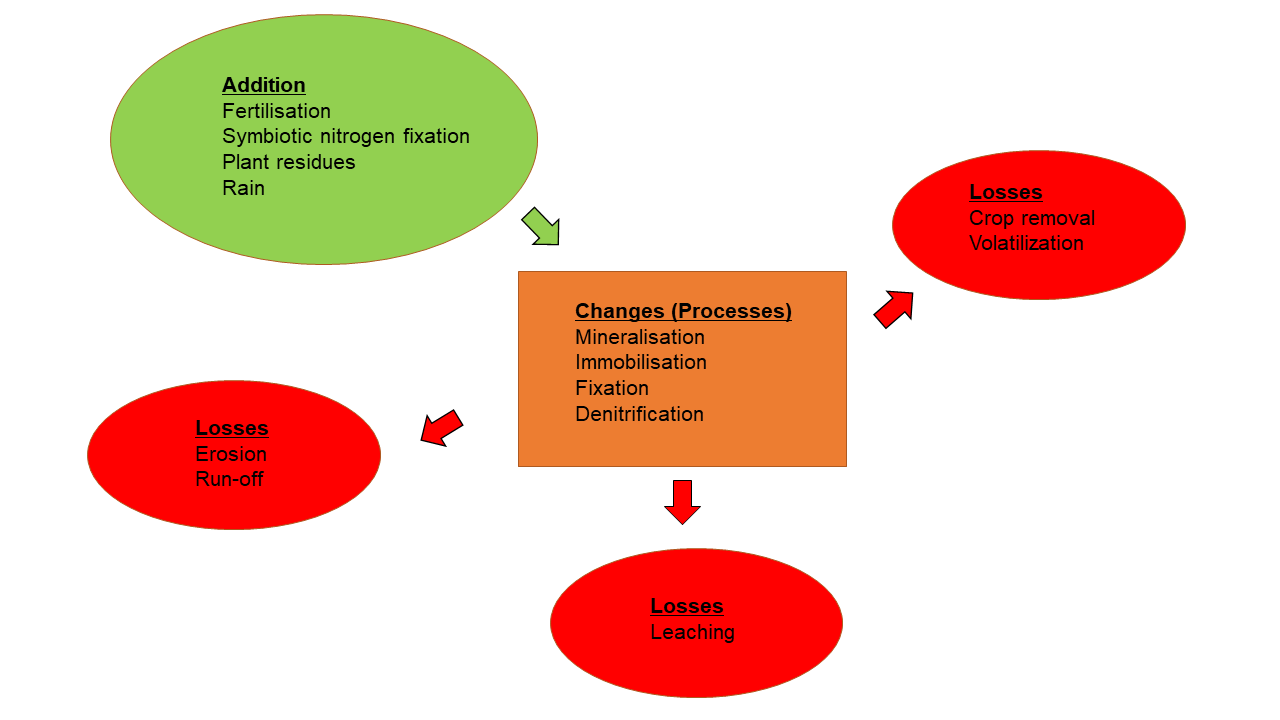
Figure 2: Additions, changes, and losses of the nitrogen cycle (from Schmidt, 1993).
With the cycle depicted and described, it is essential to understand that all nitrogen sources follow the same path; it just depends on the type of nitrogen, and where it enters the nitrogen cycle after application. In terms of plant uptake, uptake occurs as both ammonium and nitrate.
Most inorganic nitrogen (ammonium and nitrate) that remains in the soil and is not taken up by plants is eventually converted to nitrate; nitrate is therefore “Rome”, and the last plant-absorbable form in the soil; and also most subject to leaching.
The question is often asked about how long a nitrogen product takes, from application, until all the nitrogen is nitrified. According to research in the USA, the equivalent of 100 kg N/ha was mixed as urea granules into a sandy loam soil, moistened with minimum water and left to stand over a period of four weeks. All nitrogen was found to have nitrified to nitrate after four weeks (Goos, 2011).
The purpose of the preceding illustrations is to show that losses occur at any nitrogen carrier regardless. Losses are caused, apart from type of fertiliser and placement, also by soil chemical properties, occurrence of dew or water (rain or irrigation) after placement, as well as the amount of water, ambient and soil temperature, wind, water properties (in the case of irrigation), as well as aeration.
In practices where nitrogen is broadcast or left in band application on top of the soil surface, the highest degree of volatilization is observed. In such circumstances where urea lies on the soil surface for an unknown time, soil moisture or dew begins to dissolve the granules; in other words, hydrolysis begins to take place. The process of conversions begins and the pH in and around the “grain” increases, and ammonia is released in the process.
The highest degree of volatilisation occurs in high pH soils (>8,0 = 50% loss of N). Even if the soil is dried out, volatilisation can occur (Adams & Martin, 1984). In a more recent study, it is reported that more than 45% of untreated urea applied to bare soil, or soil covered with 50% straw, volatilised over a 16-day period (from an equivalent 100 kg N/ha untreated urea application (Goos, 2011).
A further risk with respect to all nitrogen carriers is the danger of seedling burn if too much nitrogen is placed too close to seed. This applies especially to untreated (non-stabilised) urea. This is why it is recommended in publications, such as the ARC Institute for Cereal Crops (Maize Information Guide – MIG), that a safe amount of nitrogen should be placed in a band 5 cm away and 5 cm deeper than seed. All excesses of this increase risk to the producer.
However, urea remains a popular choice as a nitrogen source for several reasons. It offers economic benefits in terms of manufacturing, handling, storage, and transportation. Global production of urea is high and it is the dominant nitrogen source used in the US.
Urea granules have a lower tendency to form lumps compared to calcium ammonium nitrate. In terms of storage, urea also presents a low safety risk. Furthermore, urea is less corrosive during handling in terms of equipment.
Significant savings in handling, storage, transport, and application are therefore possible due to the high nitrogen content of 46%. Calcium ammonium nitrate contains 28% N and ammonium sulphate contains 21% N. However, ammonium sulphate is totally impractical to use as a sole nitrogen source due to the low nitrogen content and the high sulphur content (24% S).
At the time of compiling this review, the approximate cost of one kilogram of nitrogen in 50 kg packages at one of Kynoch’s mixers is R39,60/kg for CAN, R37,36/kg for ammonium sulphate granules, R26,58/kg for KynoPlus, and R24,53/kg for white urea.
Take into account that international and local fertiliser prices are not always determined by actual “value”, but by supply and demand, as well as other factors. The “striking blue” KynoPlus therefore costs approximately 33% less than CAN and 7,7% more than white Urea. Now why is this important?
Worldwide, a lot of research has been done on “nitrogen stabilisers”. These “stabilisers” comprise a chemical composition (can also be a natural oil) with which white urea granules are covered. In most cases, a dye is used together with the agent to give the treated urea end product a characteristic colour.
This treatment can be a urease inhibitor, a nitrification inhibitor, or possibly a combination of the two. In the case of Kynoch, it was decided to follow the KynoPlus route; therefore a urease inhibitor. This treatment gives the original white granules a STRIKING BLUE colour. KynoPlus Active offers the user only benefits!
Results of a trial in the USA indicate great benefits with KynoPlus. The KynoPlus Active was tested against untreated urea, as well as six other ureas treated with stabilisation products on the market (Nutrisphere, Nzone, StayN, Nstay, and OAC). The results were reconstituted (Figure 3). Because the last group’s results are so close to each other, with no differences between each other, only one line is indicated in the reconstruction (Goos, 2011).
In this study, the urea pellets were left on top of the soil surface at an application rate of 100 kg N/ha. Only the Urea that was actively treated with the KynoPlus suppressed ammonia volatilization significantly.
During the same investigation, the extent to which a stabilised urea suppresses urease was measured. The active ingredient within KynoPlus actively controls the urease enzyme, which accelerates conversion to ammonium. The urease enzyme is common in nature and is produced by plants and micro-organisms in the soil, where it is found in the soil outside cells (is therefore not a living organism).
The active does not affect soil bacterial populations (EniChem, 1988) and repeated exposure to the active does not affect microbial populations or compositions (Xi et al., 2017). Urea actively stabilised (treated) with the KynoPlus significantly suppressed urease, down to as little as 2 mg/kg. The other products had no effect on urease, up to as high as 200 mg/kg.
Figure 3: Ammonia volatization for untreated and treated urea
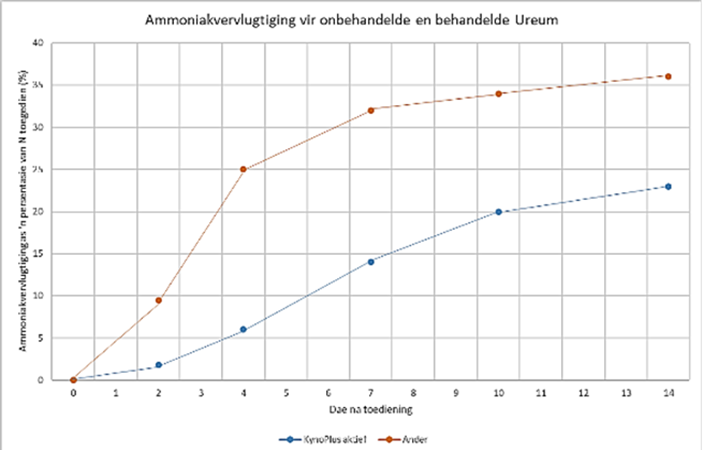
Y-axes: Ammonia volatization as a percentage of N applied (%): X-axes: Days after application
Figure 3: The percentage of ammonia that volatilised after urea was actively treated (stabilised) with the KynoPlus, versus untreated urea, as well as six other ureas treated with stabilisation products on the market (Nutrisphere, Nzone, StayN, Nstay and Nstay, and OAC ). The last group’s results are close together with no differences between them, and for that reason one line is used in the reconstruction of the trial data (from Goos, 2011).
With over 25 years of global research, with trials laid out and conducted, the KynoPlus Active is the most researched urease inhibitor trusted by users worldwide. It contains NBPT, which is approved by the American Association of Plant Nutrition Control (AAPFCO).
It is registered and approved for use in 27 countries in the European Union. It was first sold in Brazil during 2004 and since then millions of tons of urea have been treated with it worldwide. The benefits for the grower are therefore packaged as a STRIKING BLUE product that is safe to use at safe placement distances from seed (as for CAN), with reduced volatilization. It is safer to use, is less corrosive, has a high nitrogen content of 46%, and is just more cost-effective!

Figure 4: KynoPlus is supplied to the market in neat packaging (Photo credit C. Horn).
Figure 4 (photo) shows the neat packaging in which KynoPlus is delivered to the market. In Figure 5 a STRIKING-BLUE KynoPlus pellet is shown on the ground after it has been spread. In Figure 6 KynoPlus is shown on its way to being applied.
- Figure 5: The striking blue KynoPlus pellet on the ground after it has been spread (photo credit J. Sparrow).
- Figure 6: STRIKING BLUE KynoPlus on its way to being applied. (Photo credit J. Sparrow).
| You can view this product on Agri4All.com. |
References
Adams & Martin, 1984. Liming effects on nitrogen use and efficiency. In: Nitrogen in crop production. American Society of Agronomy, Madison, Wisconsin.
EniChem, 1988. Effect of NBPT on Soil Ecology, Memo on Registration of NBPT, EPA Submission.
Goos, 2011. Nitrogen fertilizer additives, which ones’ work? North Central Extension-Industry Soil Fertility Conference. November 16-17, 2011. Vol. 27. Des Moines, IA.
Havlin, Beaton, Tisdale & Nelson, 1999. Soil fertility and fertilizers. 6th edition. Prentice Hall, New Jersey, VSA.
Schmidt, 1993. Kwantifisering van die stikstofbalans op die GlenShorrocks gewasekotoop. Universiteit van die Vrystaat.
Xi, Long, Huang & Yao. 2017. pH rather than nitrification and urease inhibitors determines the community of ammonia oxidizers in a vegetable soil. AMB Express, November, Volume 7, Issue 1, Pages 1-14.











