Salmonella is an enterobacterium, which means it can primarily infect the gastrointestinal tract, although in some cases it can distribute from the intestines to other organs. Each bacterium is in a capsule, which protects it from the immune system of the host. Some of them also have flagella, which are whip-like structures that assist the bacterium movement. Both the capsule and flagella can stimulate the immune system of the host to produce an immune response against salmonella. The differences in the structure of capsule and flagella can be used to classify salmonella into different groups or serovars, which is very useful for vets and physicians to diagnose the disease and choose the correct vaccines.
Some salmonellas are host adapted, which means that they can produce disease only in a given animal species. The adaptation comes with the ability of the salmonella to spread to different organs and produce severe disease. This is the case with Salmonella gallinarum (SG) and Salmonella pullorum (SP), which affect only chickens and are unable to infect humans. At the same time, there are salmonellas that are adapted to humans, such as Salmonella typhi, which produce severe systemic infections in people, but do not affect other mammals or birds.
There are also salmonella serovars which are partially adapted or nonadapted to a given host. This means that they can infect different host species, including humans. They will be the topic of our next article. For now, we shall focus on the serovars of salmonella adapted to chickens, that is SG and SP.
Are SG and SP found in Zambia and other countries of the region?
As mentioned above, Salmonella gallinarum (SG) and Salmonella pullorum (SP) produce infections only in chickens. Infections with SG produce fowl typhoid, whilst SP results on pullorum disease. The severity of lesions and signs depend on factors such as the age of the birds affected, their breed, immune status, et cetera. SG can affect both young and adult birds, whilst SP causes infections mostly in younger flocks.
In countries with a well-developed poultry industry, fowl typhoid and pullorum disease have been under control for decades and they have not been reported lately. In Zambia and other countries of the Southern, Central and Eastern African regions, such diseases are still a problem affecting mainly layer operations. It is difficult to estimate their actual prevalence, since sometimes they go undiagnosed, and other times clinical diagnosis is not confirmed with laboratory tests.
What do we see when young pullets get infected?
SG and SP can be transmitted vertically through the egg, from infected hens to the chicks. The disease can initially manifest around hatching time or immediately after. Chicks are depressed, with no appetite, sleepy, and with adherence of chalky faeces on the feathers around the cloaca. Mortality sets in soon.
Sometimes, pullorum disease appears between the second and third weeks of age. In such cases, there is also horizontal transmission between birds of the same age. Chickens huddle close to the heat sources and have droopy wings. The flock shows difficulty to thrive. When the lungs are affected, chickens show respiratory distress (gasping). Birds that recover from the infection will never prosper, they will show poor feathering, and they may become carriers of the disease. Birds can also become blind. Sometimes, pullets develop chronic infection of the joints, especially hocks, which shows as lameness and swelling of the affected areas.
Mortality is variable, and it depends on factors such as age, breed, management, concurrent diseases, et cetera. It can be very low or reach almost 100% of the flock.
At early age, post-mortem lesions consist of congestion of the liver, spleen, and kidneys. The yolk sac contents may or may not be affected, and sometimes retention of the yolk sac occurs. In later infections, it is possible to observe nodules in the liver, lungs, and heart. Peritonitis can also be detected.
What do we see when developing pullets and laying hens are infected?
In developing pullets and adult birds, fowl typhoid (SG) is the biggest challenge, as infections with SP become less common as the birds mature.
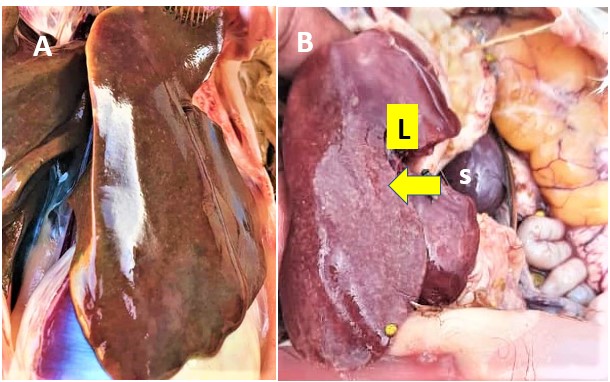
Figure 1A: Enlarged liver (hepatomegaly) in 15 weeks-old commercial pullet. Notice the typical discolouration and pinpoint necrotic foci. Flock tested positive to Salmonella gallinarum (Uganda, 2022; Dr M Banga). B: Enlarged, fragile liver (L) with areas of severe necrosis (yellow arrow) in an adult hen. Enlarged spleen (S), also presenting necrotic (white) foci (Zambia, 2022; Dr A Singole).
When the birds have already suffered the disease in early stages of their lives, it is difficult to determine which ones are carriers, since normally no signs are observed. In the onset of infection, there is a decrease in feed intake, depression, ruffled feathers, and pale combs. Diarrhoea can be seen. There may be a reduction in egg production. Mortality is variable, and some chronic cases show moderate daily mortality that can continue for a prolonged period.
Liver lesions are typical post-mortem findings. The liver is enlarged, fragile, of a brown/bronze colour, with areas of necrosis (Figure 1A). The spleen is generally enlarged (Figure 1B).
In mature layers that are carriers, there may be regressed ovarian follicles with abnormal colour and thickened walls. Internal ovulations can be observed, with subsequent peritonitis. In some cases, yolks get impacted in the oviduct, forming large masses of hardened material.
There may also be inflammation of the membranes covering the heart and the liver.
How do I treat birds infected with salmonella?
Although birds recover when the right antibiotic at the right dose is administered, one should be aware that the flock will never be salmonella free. In fact, the infection sometimes returns shortly after finishing the antibiotic treatment. This is because salmonella has the ability of colonising cells of the immune system in which they remain dormant; when this occurs, the birds become carriers. Once the carriers face a stressing situation (for example another disease, overcrowding, et cetera), the infection reactivates, and the carriers shed salmonella to the environment once again. The carrier birds may or may not develop symptoms, but new cases of the disease begin to appear due to horizontal transmission.
How do we prevent salmonella infections?
Prevention is key for the control of the disease since, once the bacteria enter the flock, it is impossible to eradicate it unless the farm is depopulated and left to rest. Prevention is based on reducing the amount of salmonella in the environment by applying appropriate biosecurity measures, good hygiene, and good management practices. For example, chemical rodent and insect control has a large impact in reducing the chances of infection, since rats and mites are involved in the transmission of the disease.
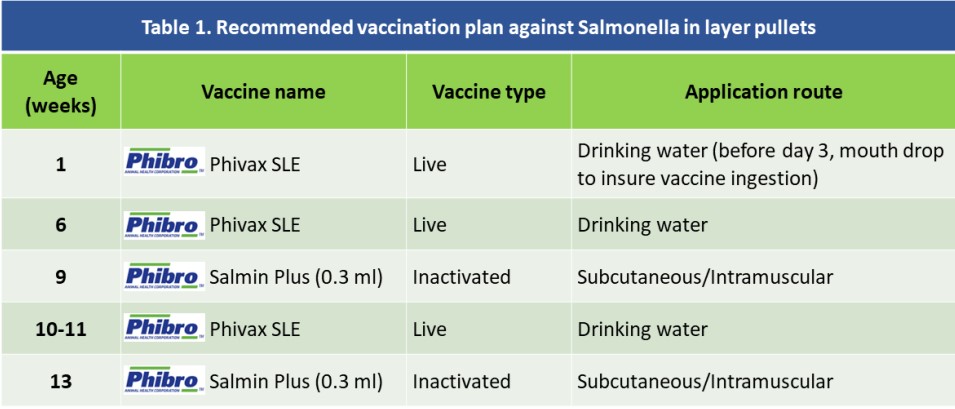
Vaccines play an important role in preventing infections, however we cannot expect good protection if their application is not supported by proper biosecurity and management measures. Phibro has an integral control programme against SG, SP and other salmonella serovars, based on the administration of the live vaccine Phivax SLE (applied early in life), followed by the inoculation of an inactivated vaccine (Salmin Plus) later in rearing (Table 1).
We shall discuss prevention in depth in the next article of this series.
As usual, please consult your veterinarian about salmonella infections and protection before making any changes at your farm.











have already been following your site for several days. absolutely love what you posted. btw i will be conducting a study relating to this topic. do you know other great websites or maybe forums in which I can get more information? thanks in advance.