Infectious Bronchitis virus (IBV) variant 2 has been detected in Zambia by PCR and genetic sequencing. Although the number of cases is on the rise and the number of flocks sampled is increasing, we believe the disease is still underdiagnosed. During post-mortem examinations, we observed the occurrence of cystic oviducts.
They can often be linked to IB variant 2 infections that could have occurred during the first weeks in the life of the pullet. Although such early infections are normally not accompanied by high mortality rates, the multiplication of the IB variant 2 virus in the oviduct results in abnormal development of this organ, with subsequent thinning of the wall, accumulation of fluid, and formation of cysts (Figure 1). When birds with cysts reach sexual maturity, the progression of the yolk through the oviduct towards the uterus becomes impossible and the egg cannot be formed.
Oviductal cysts are an incidental finding during post-mortem examinations. It is impossible to detect birds with cysts by visually inspecting the flock unless the cysts are so large that the abdomen is distended and the bird needs to stand upright to be able to breathe (penguin posture). Affected birds are usually called false layers, since they look sexually mature, and eat their daily ration, but do not produce eggs. If there are a significant number of false layers, it will manifest as a healthy flock with sustained poor production levels.
Flocks that suffer early infection can also be reinfected later if proper preventative measures (including appropriate vaccination) are not implemented. It is opportune to mention that when Variant 2 infections occur during the late rearing and laying stages, the disease profile will be more severe and may result in higher mortalities, significant drops in egg production, as well as problems of eggshell quality (discolouration, brittle shells, shell-less eggs, etc.). How to prevent early infections in pullets? The most effective strategy to prevent IBV variant 2 infections in pullets and their associated production losses consists of implementing a vaccination program with TAbic IB VAR206. This vaccine (developed from the IBV Variant 2 field strain) has shown to be highly effective in controlling field challenges by Variant 2 (as well as some other commonly circulating field strains), provided it is applied correctly.
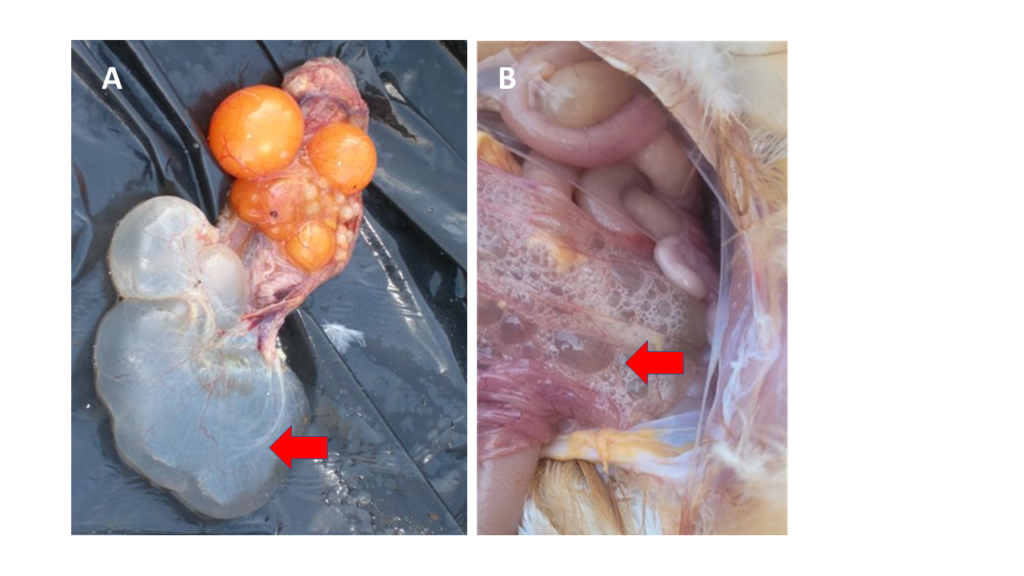
Figure 1. Two examples of oviductal cysts in layers (red arrows). In both, the oviduct presents very thin walls (atrophied) and it is filled with fluid. Picture B was taken in Lusaka (September 2022) in a 42 weeks-old bird from a layer flock positive to IBV variant 2.
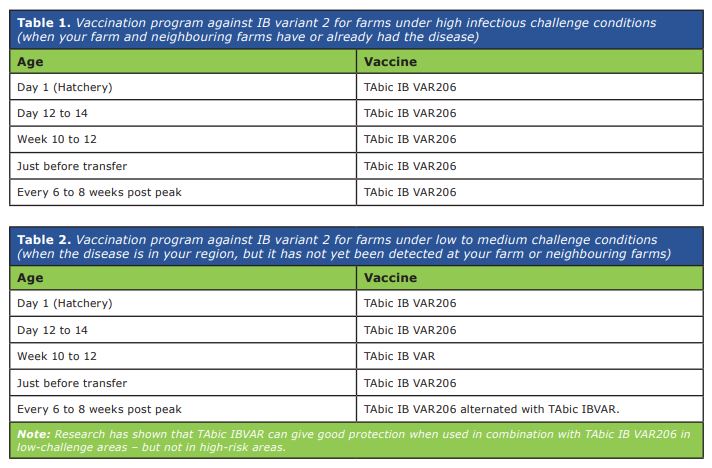
It is necessary to remark that the inclusion of TAbic IB VAR206 at the hatchery is essential to minimise the chances of early infections in pullets, which are not unusual in Zambia. Hatcheries may be concerned about the possible spreading of IB vaccines (including TAbic IB VAR206) between chick boxes after hatchery vaccination, which could result in rolling reactions among chicks. However, studies have determined that such post-vaccination spreading does not occur, even if the boxes are very close to each other. Tables 1 and 2 show two recommended vaccination plans to prevent infections by IB variant 2.
The choice between them should be based on the risk of infection at the farm. As usual, inactivated (injectable) vaccines should be also included in the rearing vaccination program, according to local epidemiological situation. Whilst spray vaccination stimulates immunity mainly at the points of entry of the virus (eyes, nostrils, trachea), inactivated vaccines are essential for the generation of circulating immunity. TAbic IBVar 206 can be combined in the same vaccination procedure with other IB vaccines (e.g. TAbic IB VAR) and with VH (against Newcastle disease), provided that no more than three vaccines are mixed at one time.
Some extra tips to improve vaccine effectiveness Remember that, in order to maximise vaccination effectiveness:
1. The water used for preparing the vaccine should be clean, free of chlorine and have a pH between 6-7.5. To achieve this, you can add a commercially available chlorine neutralizing product to the water before adding the vaccine. Furthermore, the addition of some colourants will help you to evaluate the success of the vaccine application.
2. It is very important to ensure that the temperature of the water used in the vaccination process is kept between 6°C and 10° C as far as possible. For every degree above 10° C, the vaccine losses 3.3% effectiveness. Therefore, if the vaccine water is at 25° C, the vaccine will be (in the best of cases) 50% less effective.
3. Apply IB vaccine only in a spray. The effectiveness of IB vaccines in drinking water is low and unpredictable. As always, please consult your veterinarian before making any changes in your vaccination program.









